2.
Photo-fermentation and photolysis by microalgae
General principle of hydrogen photoproduction by microalgae
Green unicellular algae are eukaryotic cells in which
the chloroplast performs the photosynthetic assimilation and reduction of CO2,
to synthesize mainly carbohydrates. This function is realized through the
conversion of light energy, absorbed by photosystems, into redox energy (NADP
reduction) and phosphate potential (ADP phosphorylation), which are then used in
the process of CO2 assimilation and reduction through the Calvin
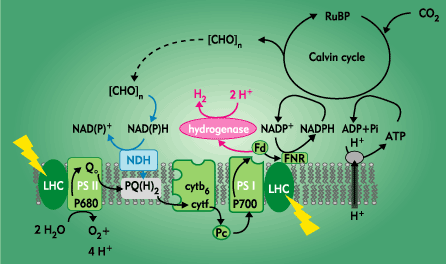
cycle (Figure 1).
Two multi-proteic complexes, the photosystems (PSI and PSII), are embedded in the internal thylakoid membranes of the chloroplast and harbour several hundreds of chlorophyll pigments each. The absorbed light energy is used to drive electron transport from water to ferredoxin. This water-soluble protein can then be used for the reduction of NADP. Electrons are first transferred by PSII from water to a pool of plastoquinones. A cytochrome complex (Cyt b6/f) catalyzes electron transport from plastoquinones to plastocyanins. The electrons are then further light-activated by PSI to be transferred to ferredoxin. During the process of electron transport, a trans-thylakoidal electrochemical gradient is formed, which drives ADP phosphorylation catalyzed by a chloroplastic ATP-synthase. All these reactions constitute the so-called ‘light phase’ of photosynthesis. In aerobic conditions, the reducing power generated by this process is then used for CO2 reduction.
In anaerobic conditions, some green unicellular algae
synthesize hydrogenases. In the chloroplast, these enzymes catalyse the
reduction of protons by ferredoxin with formation of molecular hydrogen (H2).
Hydrogen photoproduction is then observed: light energy then promotes an
electron transport process, which sums up to a splitting of water into oxygen
and hydrogen. This phenomenon was discovered in the thirties by Hans Gaffron and
co-workers, who showed that several species of green unicellular algae started
to evolve hydrogen in the light after they were adapted to anaerobiosis (Gaffron
and Rubin 1942, Stuart and Gaffron 1972). In recent research, this phenomenon
has mainly been studied in Chlamydomonas reinhardtii.
Two hydrogenases were discovered in this microalgae. They belong to a
family of Fe-containing hydrogenases. Fe-hydogenases have high activities
compared to other hydrogenases, such as Ni-Fe or non-metal hydrogenases of
bacteria (Happe et al. 1994, Happe et al. 2002). Oxygen prevents the expression
of hydrogenase genes, and inhibits enzymatic activity.
|
Figure 1
(Verméglio et al.,
http://www.cea.fr/Fr/Publications).
Photosynthetic electron
transport and hydrogen production in green unicellular
microalgae.
PSI: photosystem I, PSII:
photosystem II, lhc: light-harvesting complexes, Q/PQ:
plastoquinones, cyt: cytochromes, Pc: plastocyanins, Fd:
ferredoxin, FNR: ferredoxin-NADP oxidoreductase, NDH:
chloroplastic NAD(P)H dehydrogenase |
The complexity of energetic metabolism linked to
hydrogen photoproduction in Chlamydomonas reinhardtii
Already in the 70’s, it was recognized that the metabolic pathways that participate to (or interact with) H2 photoproduction are complex. For instance, photoproduction is only partly inhibited by a strong PSII inhibitor (Stuart and Gaffron 1972). One must therefore hypothesize that electrons can be somehow ‘injected’ in the electron transport chain by a lateral pathway (Figure 1). Further studies have demonstrated the occurrence, in green microalgae, of a chloroplastic NAD(P)H-dehydrogenase : NDH (reviewed by Peltier and Cournac, 2000). This enzyme would catalyse a non-photochemical (PSII-independent) reduction of the plastoquinones. It would use NADPH or NADH formed during oxidative metabolism. The occurrence of this pathway explains that H2 evolution can occur when PSII is inactive. The required electrons then originate from oxidation of cellular metabolites. In green microalgae, enzymes of glycolysis and oxidative pentose pathway are mainly localized in the chloroplast (Chen and Gibbs, 1991). Starch, synthesized as a result of photosynthesis, can thus be degraded in the chloroplast and generate NAD(P)H to fuel the H2 production process. Only PSI is then implicated in the light-activation of electrons for their transfer to hydrogenases.
From the above description, it follows that H2
evolution by green microalgae is partly a photofermentation process, which
allows NAD(P)H oxidation for continuous operation of oxidative metabolism. The
coupling of NAD(P)H oxidation to the reduction of the chloroplastic electron
transport chain results in the beneficial ATP generation, which contributes to
cell survival during anaerobiosis. Hydrogen can thus be viewed as a
photofermentation by-product. Small carbonated metabolites such as formate,
ethanol and acetate are other fermentation products in Chlamydomonas,
whose unusual fermentation processes have been recently investigated
(Hemschemeier and Happe, 2004). Besides this photofermentation process,
PSII-dependent electron transport form water to ferredoxin also contributes to H2
evolution under anaerobiosis (Antal et al. 2001).
Mitochondrial respiration also fulfils an important
function during H2 photoproduction under anaerobiosis. Oxygen evolved
in the chloroplast due to water-splitting at PSII can be eliminated in the
mitochondria. However, the ability of mitochondria to efficiently scavenge
oxygen is limited and H2 evolution usually stops when PSII activity
is high, due to the inhibition of hydrogenases by oxygen.
In the first studies on microalgal hydrogen metabolism, high yields of H2 evolution in the light were only transiently observed. Within a short time after the onset of illumination, hydrogenases are inhibited by oxygen produced at PSII and H2 evolution stops. Simultaneous production of hydrogen and oxygen can only be stabilized by continuously eliminating oxygen, for instance by flushing helium through the medium (Greenbaum 1980). Very high quantum yields of 1 H2 molecule per 4 absorbed photons could be achieved in such conditions (Greenbaum 1988).
Recent progress towards sustained hydrogen photoproduction by Chlamydomonas reinhardtii
The incompatibility between oxygen and hydrogen evolution remained problematic until it was found that alterations of the metabolism caused by sulfur deficiency could be exploited for sustained H2 photoproduction by Chlamydomonas (Melis et al. 2000). In S-free medium, PSII function is greatly decreased due to slow turn-over of the reaction center proteins. In addition, starch accumulation is favoured. These effects are beneficial for H2 photoproduction through the photofermentation pathway. After adaptation to S-free medium, anareobiosis is reached in the light due to sustained respiration and decreasing photosynthetic oxygen evolution. H2 production is then observed in the light during three-four days. Then, the algae have consumed most of their reserves and need to be transferred back to complete medium. Several cycles of S-free and S-replete cultivation steps can be repeated with good reproducibility of the hydrogen evolution rates (Melis 2002). This experimental system has been termed the ‘two-stage photosynthesis and H2-production process’. It provides a good experimental procedure for fundamental studies on the hydrogen metabolism and the photosynthesis-respiration interactions. It is also a starting point for the design of improved procedures for economically viable H2 photoproduction by Chlamydomonas
.
Increasing the yield of H2 photoproduction in the two-stage process: a challenge for research
Reported rates of H2 photoproduction in the
two-stage system are in the order of 2 ml H2 per liter of algal
suspension per hour. The biogas is almost (98%) pure H2 with only
traces of CO2 and other gases. Taking into account known records of
maximum photosynthetic efficiency, it can be concluded that the observed H2
yield is weak compared to the theoretical maximum (Melis and Happe 2001). This
has stimulated recent research aiming at understanding limiting factors in the
process and improving yields through genetic approaches. There are probably
several reasons for the low hydrogen photoproduction rates:
Under sulfur deficiency, the activity of PSII is weak. This allows the
maintenance of the activity of the hydrogenases, but at the same time it leaves
the photofermentative pathway as the predominant contribution to H2
formation. It is likely that in such conditions the rate of plastoquinone
reduction, catalysed by NDH, limits the input of electrons in the photosynthetic
electron transport chain (Cournac et al. 2002).
-The formation of a trans-thylakoidal electrochemical proton gradient
opposes to the electron transport process. This has been shown for algal cells
grown on complete medium by the use of uncoupler, the addition of which strongly
stimulates H2 photoproduction (Cournac et al. 2002). It is not known,
however, if limitation by the electrochemical gradient also occurs in
S-deficient cells.
-S deficiency affects protein synthesis and turn-over, including the
synthesis of hydrogenases (Zhang et al. 2002)
-The quantum yield of H2
photoproduction decreases at high
light intensity because an increasing part of the absorbed energy is dissipated
as heat due to a phenomenon known as non-photochemical quenching. This takes
place mainly in the light-harvesting pigment-protein complexes, LHCII.
The search for genetic mutants with increased H2
photoproduction capacities has begun. Genetic modification of the Fe-hydrogenases
in order to make them less sensitive to oxygen, has only met limited success.
Some improvement of the H2 rates has been obtained in mutants with
altered pigmentation of the photosystems (Melis 2003). Surprisingly, the most
obvious improvement of H2 photoproduction in mutant strains has been
described for a mutant, which is altered in its mitochondrial functions (Kruse
et al. 2005). The exact reason for the observed improvement in this mutant is,
however, not well understood as chloroplastic functions are also modified in the
mutant. As a whole, these results hold promise of significant improvements of H2
photoproduction in Chlamydomonas reinhardtii by the genetic approach (Melis
et al. 2004).
A new direction in bio-hydrogen production systems is
the use of mixed cultures of micro-algae (Chlamydomonas reinhardtii) and
photosynthetic, hydrogen producing bacteria such as Rhodospirillum rubrum,
followed by the fermentation of biomass by Clostridium for additional H2
production (Melis and Melnicki, 2006). It is expected that the combination of
complementary metabolisms and complementary light absorption spectra may
contribute to significantly increased efficiencies of mixed systems compared to
monocultures.